The FDA User Fees for FY 2024, October 1, 2023 – September 30, 2024, were released on Friday, July 28, 2023.
What are FDA User Fees?
At the very core of it, the FDA user fees fund the FDA Office of Device Evaluation (ODE) budget. Without these user fees, the FDA cannot begin reviewing a medical device submission. This includes 510k, PMA, and De Novo submissions. Before the FDA assigns a reviewer to your submission, you must pay the appropriate device user fee in full unless eligible for a waiver or exemption. If you pay the user fee by credit card, you must allow a few extra days for the user fee to clear. Otherwise, your submission will be placed on “User Fee Hold.” Small businesses may qualify for a reduced fee. The FDA announced the FY 2024 FDA User Fees on July 28, 2023. The FDA will announce the user fees for FY 2025 in a Federal Register notice next August 2024.
What are the FDA User Fees for FY 2024?
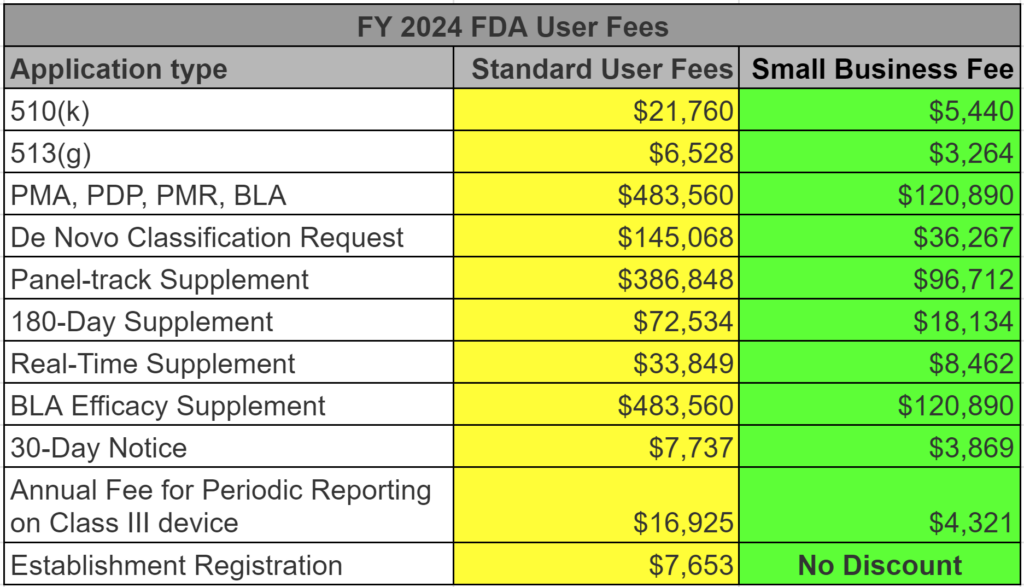
How much did user fees increase for FY 2024?
The increase in FDA user fees from FY 2023 to FY 2024 was 9.5%, except the annual FDA Registration fee, which increased by 17.87% to $7,653. There are three components to the increase:
- Base Fee = a statutory base fee for each FDA user fee
- Standard Fee = an inflation-adjusted statutory base fee
- Adjusted Fee = adjusted fee to meet revenue target
The reason for each component for the user fees is described in the Federal Register.
When does the FY 2024 increase take effect?
Each year the new FDA user fees take effect on the 1st day of the FDA’s new fiscal year (i.e., October 1). You cannot pay the annual registration fee for FY 2024 until October 1, 2024, and the last day you can submit under the FY 2023 user fee pricing is Friday, September 29, 2023. For the submission to be accepted under the current fiscal year, the submission must be uploaded to the Customer Collaboration Portal (CCP) or received by the Document Control Center (DCC) via courier no later than 4:00 pm EDT on the 29th.
What do you do if you have already paid the FY 2023 price?
If you already paid the FY 2023, and your submission is received after 4:00 pm EDT on September 29, 2023, you must complete FDA Form 3914 for an FDA user fee payment transfer request. You will also need to pay the difference in user fees (i.e., 9.5%). If your submission is received before the FY 2023 user fee is transferred and you have paid the difference in user fees, your submission will be placed on a user fee hold. If you paid the FY 2023 user fee and are not ready to transfer your previously paid user fee to FY 2024 (and pay the difference), you can request an FDA user fee refund by filling in an online form.
What is the annual registration fee for FY 2024 due?
The annual establishment registration user fee can be paid any time between October 1 and December 31. If you pay late, there is no penalty, but your registration status will be inactive, and you cannot submit new device submissions or import products to the USA. If you are not yet distributing any devices in the USA, you are not required to have your establishment registered, and establishment registration is not required before submitting a new device submission. If you are not required to register yet, when you are paying the user fee for a new device submission on the Device Facility User Fee (DFUF) website, you will click the “Yes” button because there is no “N/A” option for the question below.
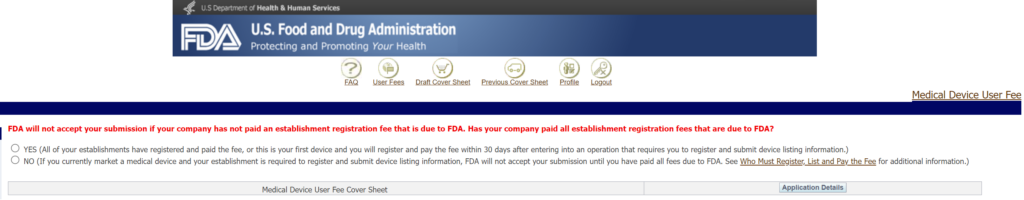
Is the annual FDA registration fee prorated?
Annual registration payments are not prorated when you are paying in the middle or even near the end of the year for your initial registration. Therefore, you will need to consider if the revenues you expect to gain before the end of the current fiscal year are worth the registration cost. If you need any help with annual registration or you need a US Agent, we offer these consulting services.

Pingback: How much does a 510k cost? - Medical Device Academy Medical Device Academy
Pingback: FDA Registration and Listing for Medical Devices Medical Device Academy
Pingback: What is MDUFA V? - FDA - Medical Device Academy Medical Device Academy
Pingback: How long does it take FDA to review De Novo submissions? Medical Device Academy
Pingback: What is an FDA Breakthrough Device Designation? Medical Device Academy
Pingback: FDA US Agent - What do they do? - Medical Device Academy Medical Device Academy
Pingback: HDE Application and 510k Submissions Medical Device Academy
Pingback: Four easy ways 510k and De Novo content is different Medical Device Academy